Fit Test Colon Cancer Sensitivity
The aim of this study was to assess the use of FIT within the recent NG12 and DG30 National Institute for. A new study published in the journal gut examines the accuracy of fit as a predictive tool for colorectal cancer.
Https Www Cdphp Com Media Files Providers Toolkits Colorectal Cancer Colorectal Cancer Screening With Fit Vs Cologuard Pdf
Fit Test Colon Cancer Sensitivity.

Fit test colon cancer sensitivity. Background Aims. The FIT-DNA test also referred to as the stool DNA test combines the FIT with a test that detects altered DNA in the stool. FIT stands for fecal immunochemical test so the proper terminology is just FIT instead of FIT test since test is already a part of the FIT acronym.
Objective To assess the diagnostic accuracy of FIT for CRC or advanced neoplasia AN in. This type of screening test is designed to detect hidden blood in a stool sample. Though it is often cited that Cologuard has a sensitivity of 92 compared with FITs sensitivity of 74 it is important to.
070 ci 055 to 081 at cutoff values of 20 to 50 gg but with a corresponding decrease in specificity. The sensitivity of the fecal occult blood test by FIT was significantly higher for stages III and IV colorectal cancer than for stages I and II P001 and it was insignificant for the guaiac fecal occult blood test P007. The diagnostic sensitivity of FIT testing may vary depending on lesion location in the colon.
Diagnostic performance of FITs depends on the cutoff value for a positive test result. FITs detect most CRCs. Objective Faecal immunochemical test FIT shows promise as a non-invasive triage test for colorectal cancer CRC in the symptomatic population.
Blood vessels at the surface of larger polyps or cancers are often fragile and easily damaged by passing stool. Fecal immunochemical tests FITs detect the majority of colorectal cancers CRCs but evidence for variation in sensitivity according to the CRC stage is sparse and has not yet been systematically synthesized. This detection is important because it can be a sign of precancerous polyps or colorectal cancer.
Although detection of CRC at early stages is most relevant for reducing CRC mortality there is limited evidence for the stage-specific sensitivity of the FIT in CRC detection. Fecal immunochemical tests are moderately sensitive are highly specific and have high overall diagnostic accuracy for detecting CRC. Fecal immunochemical tests FITs detect the majority of colorectal cancers CRCs but evidence for variation in sensitivity according to the CRC stage is sparse and has not yet been systematically synthesized.
Tumors were stratified by location. The evidence generated from the study shows that FIT. The fecal immunochemical test FIT also called an immunochemical fecal occult blood test iFOBT is a newer kind of stool test that also detects occult hidden blood in the stool.
It is done once every three. Like the guaiac faecal occult blood test gFOBT FIT detects occult blood in faeces although it detects globin rather than haem making it more specific for human blood17 FIT is replacing gFOBT in CRC screening programmes because of its ease of use increased uptake quantitative analysis and greater sensitivity for advanced colorectal neoplasia ACN18 19 The English Bowel Cancer Screening Programme BCSP is planning to introduce FIT. Thus our objective was to systematically review and summarize evidence on the stage-specific sensitivity of FITs.
Proximal lesions may be harder to detect for several reasons including that they may arise from serrated lesions that are flat and because they are less vascular than. Blood vessels in larger colorectal polyps or cancerous growths are much more fragile and can be easily broken by the passage of a stool causing bleeding in the colon. It is also done once a year in the same way as a gFOBT.
FIT vs Cologuard FIT-DNA Effectiveness convenience and cost are the three factors that determine which tests to pursue with a patient who is resistant to screening colonoscopy as well as flexible sigmoidoscopy or CT colonography. Sensitivity was particularly low for T1 and stage I cancers in the distal colon 32 and 52 respectively although. FIT sensitivity for colorectal cancer CRC is maximised to 970 at the limit of detection of 2g haemoglobin Hbg faeces gg.
The FIT identified patients with CRC with overall high sensitivity but missed nearly 50 of small T1 and 32 of UICC stage I CRCs. Thus our objective was to systematically review and summarize evidence on the stage-specific sensitivity of FITs. Fecal immunochemical tests FITs are widely used for colorectal cancer CRC screening.
The fecal immunochemical test FIT uses antibodies to detect blood in the stool. For this test you collect an entire bowel movement and send it to a lab where it is checked for cancer cells. The study has shown FIT to be as sensitive a test as colonoscopy which is currently the gold standard for detection of colorectal cancer.
A faecal Hb concentration f -Hb result less than the limit of detection in symptomatic patients indicates that their chances of not having CRC is 998. Importance The potential role of the fecal immunochemical test FIT for screening patients at increased risk for colorectal cancer CRC has not yet been elucidated. They used Union for International Cancer Control UICC stage and T stage infiltration to measure sensitivity.

Performance Of Two Faecal Immunochemical Tests For The Detection Of Advanced Neoplasia At Different Positivity Thresholds A Cross Sectional Study Of The Dutch National Colorectal Cancer Screening Programme The Lancet Gastroenterology

The Irreplaceable Colonoscopy Carolina Digestive

Lower Abnormal Fecal Immunochemical Test Cut Off Values Improve Detection Of Colorectal Cancer In System Level Screens Sciencedirect

Efficacy And Cost Comparison For Colorectal Cancer Screening Alternatives Download Table

The Fit Test For Bowel Cancer Screening Faecal Immunochemical Testing
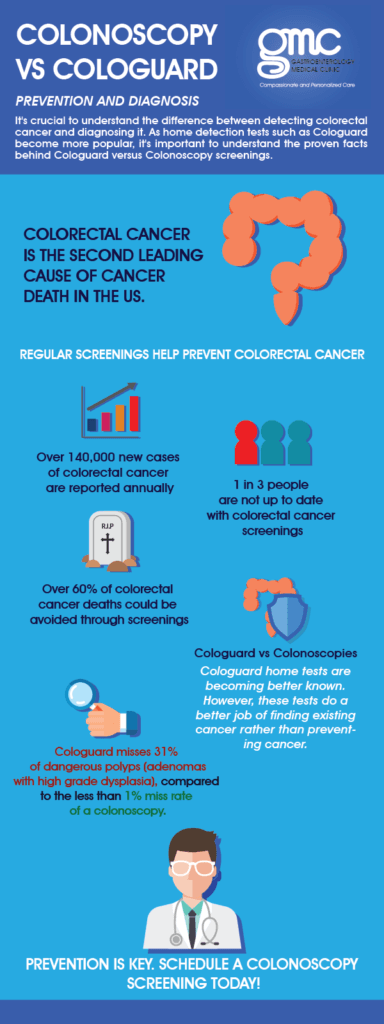
Cologuard Or Colonoscopy Prevention Vs Diagnosis Of Colorectal Cancer
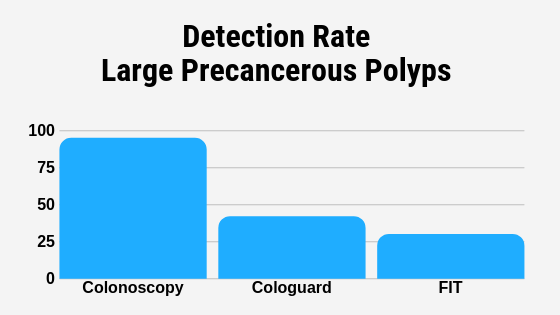
The Truth About Cologuard Tests Gastroenterologist San Antonio
Oncoprescribe Write The Perfect Prescription

Cologuard Vs Colonoscopy Atlanta Gastroenterology Associates

At Home Fit Colon Cancer Screening Test Kit Everlywell

Colon Cancer And Prevention 101 Blog Everlywell Home Health Testing Made Easy

Study Flow Diagram Fit Fecal Immunochemical Test Download Scientific Diagram
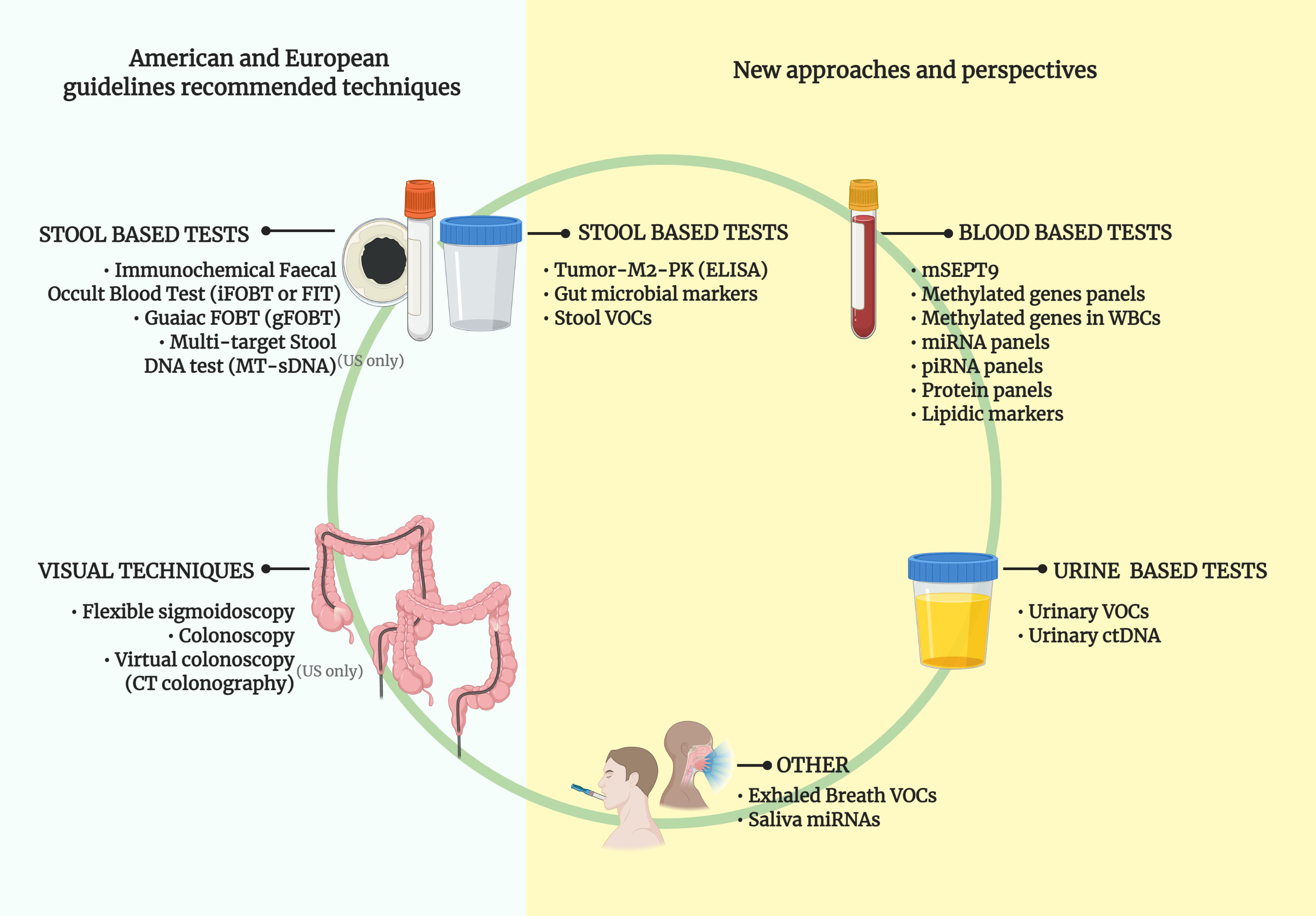
Cancers Free Full Text Towards Novel Non Invasive Colorectal Cancer Screening Methods A Comprehensive Review Html

Second Generation Fit 1 Pack Pinnacle Biolabs

Sensitivity Of Fecal Immunochemical Test For Colorectal Cancer Detection Differs According To Stage And Location Sciencedirect

Second Generation Fit 1 Pack Pinnacle Biolabs

Second Generation Fit 1 Pack Pinnacle Biolabs
For Colorectal Cancer The Only Good Screening Test Is One That Is Taken Singlera Genomics
What Is A Fit Test Blog Everlywell Home Health Testing Made Easy
Post a Comment for "Fit Test Colon Cancer Sensitivity"